Accuracy of HER2 Testing in Gastric Cancer
 With almost 1 million new cases per year, gastric cancer is currently the number four most common malignant disease. Today, there is no universal standard treatment. Recently, however, the ToGA trial demonstrated efficacy of Trastuzumab (Herceptin) for gastric and gastro-oesophageal (G/GEJ) patients who are over-expressing HER2. In 2010, the FDA approved Trastuzumab for treatment of this patient population.
With almost 1 million new cases per year, gastric cancer is currently the number four most common malignant disease. Today, there is no universal standard treatment. Recently, however, the ToGA trial demonstrated efficacy of Trastuzumab (Herceptin) for gastric and gastro-oesophageal (G/GEJ) patients who are over-expressing HER2. In 2010, the FDA approved Trastuzumab for treatment of this patient population.
As is for breast cancer, this creates a need for sensitive and specific diagnostic tools that allow pathologists to identify responders.
Diagnostic Challenges
The most commonly used approaches in routine testing are FISH and IHC. The main advantage of IHC is that it is inexpensive in terms of reagents and equipment, and typically with significantly shorter turn-around times. But there are challenges.
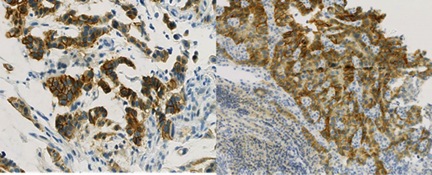
Intra-tumoral heterogeneity and very different membrane morphology relating to the incomplete membrane staining (apical portion), makes it harder to read and interpret HER2 status in patients. As a consequence, the number of 2+ cases is generally much higher, leading to increased cost and significantly longer turn-around times for patients. Also, there appears to be a higher IHC/FISH discordance rate than observed for breast.
In addition, using the testing principles established for breast cancer in gastric cancer, may result in up to 50% false negatives, according to Prof. Stephen Finn of St. James Hospital in Dublin.
Image Analysis and Membrane Morphology
For breast cancer, Visiopharm’s HER2-CONNECT method has been CE-marked for in-vitro diagnostic use. This method provides a generalized (and continuous) measure of circumferential membrane staining across tumor cells. In a European Validation Study on breast cancer, this method was shown to significantly reduce inconclusive (+2) readings with approximately 70% compared to manual reading, without loss of sensitivity or specificity with regard to gene amplification. Performance data is presented in the package insert, and today the method is in clinical use.
Based on these encouraging findings, Prof. Finn and his team wanted to explore if the connectivity principle was also applicable for quantifying HER2 protein expression or gastric cancers. They also wanted to understand how the differences in membrane morphology was reflected in the membrane connectivity; and finally whether it would be possible to achieve a similar reduction in reflex testing without compromising sensitivity/specificity with regard to gene amplification.
Join Prof. Stephen Finn next week as he presents his practical experiences and findings during the webinar, Connectivity based HER2 IHC Membrane Image Analysis in Gastric Cancer on in April 7, 2015 at 4 PM CET/ 10 AM EST /7 AM PST.
Follow Visiopharm and CEO, Michael Grunkin, on LinkedIn and read more about Visiopharm’s plan for in-vitro diagnostic use of HER2 for Gastric Cancer, other interesting topics and more.

































