Synoptic Reports for Patients
I am a big fan of synoptic reports for cancer diagnoses. They allow us to standardize our reporting of tumor site, type, grade stage, size and many other characteristics of the tumor that comprise staging information required for therapy. We can have more uniform reporting within our groups and among each other. The College of American Pathologists (CAP) and many of its partners have worked diligently over the past several years to refine these over time and keep them up to date as well as provide additional enhancements to laboratory information systems for best clinical practices. Worksheets, templates, drag and drop menus, fillable fields, etc… all help to insure the necessary, and if desired, optional data elements are reported for patient care. The separate data fields allows for later mining based on a specific grade, stage, site, size, age, biomarker profile, etc… than many systems we are familiar with would allow with textual data alone.
 Each organ of course is a little different. There have been many changes to the colon template, for example, with molecular ancillary studies and other findings that may not have been commonly performed or mentioned in the patient report. Recently I used the thyroid and prostate worksheets to complete reports, the thyroid seemed longer with more data elements while prostate seems to remain more lean with weight, size, Gleason grade, margins, extraprostatic disease, lymphvascular space, margin and/or seminal vesicle involvement that seem to make sense and are what I recall needing to report as a resident 20 years ago.
Each organ of course is a little different. There have been many changes to the colon template, for example, with molecular ancillary studies and other findings that may not have been commonly performed or mentioned in the patient report. Recently I used the thyroid and prostate worksheets to complete reports, the thyroid seemed longer with more data elements while prostate seems to remain more lean with weight, size, Gleason grade, margins, extraprostatic disease, lymphvascular space, margin and/or seminal vesicle involvement that seem to make sense and are what I recall needing to report as a resident 20 years ago.
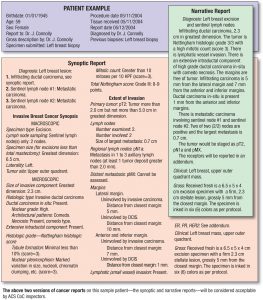
How does a patient interpret these reports? Some are better than others but often times the words “adenocarcinoma” or “papillary type, usual (classic) variant)” may not appear until the 5th or 6th line in the summary. How are they to take these reports and make sense of what may be significant or not and how is their disease different than others with a particular type of cancer? Would “N/A” throw them off simply because there was no neoadjuvant therapy prior to surgery or how are they to know it is “not applicable” to them?
I realize the reports are for surgical, medical and radiation oncologists but I think pathology has an opportunity and responsibility to create patient-centric reports. Imagine if you will, the ability to convert a pT3b combined Gleason grade 7 to a report for a patient with information relevant to them.
Perhaps embedded links for “How patients with similar grade and stage tumors were treated at this instution” or “What additional therapies might be discussed with you and how you might be followed following your surgery”. Perhaps based on the taxonomy in the synoptic report, rules could be created in the report about “Thyroid cancer support groups in your community” or “What every patient with melanoma needs to know about their pathology report” and highlight importance of depth, type, regression, etc… in terms patients can understand if they choose to read the information.
We as gatekeepers of this data should be able to use this information not only for our clinical colleagues but our patients, our customers and provide a service to them beyond what we provide to their doctors alone.

































