Philips receives FDA clearance for Digital Manual Read of HER2 pathology slides
- Philips introduces clinical digital imaging product for pathologists.
- 510(k) clearance from the US Food and Drug Administration (FDA) allows Philips to market its Her2/neu IHC Digital Manual Read product in the US.
- Pathologists can now use a digital system from Philips to help them assess treatment options for breast cancer patients.
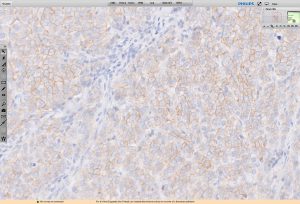 Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in healthcare and digital pathology, announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) to market its Her2/neu IHC Digital Manual Read product in the US.
Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in healthcare and digital pathology, announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) to market its Her2/neu IHC Digital Manual Read product in the US.
HercepTest™ is a commonly used test for pathologists in the assessment of treatment options for breast cancer patients, using conventional microscopy. Now, with Philips Her2/neu IHC Digital Manual Read, they asses the HER2 status using a digital system and a computer monitor.
Pathologists can now benefit from the digital imaging advancements for scoring HercepTest™ stained tumor tissue slides that are digitized by Philips ultra-fast scanner, and made accessible through Philips advanced image viewing and analysis management system.
“Digitalization will continue to revolutionize healthcare by providing the right clinical information to the right caregiver at the right time.” says Perry van Rijsingen, General Manager of Philips Digital Pathology. “Clinical pathologists will now have direct access to the digitally stored Her2/neu IHC images to help them in the detection and semi-quantitative measurement of immunohistochemically (IHC) stained breast cancer tissue on their computer monitor.” The Philips Her2/neu IHC Digital Manual Read is based on the Philips Digital Pathology Solution platform. This automated digital slide creation system was commercially introduced in Europe and Asia Pacific in 2012. With the FDA clearance, Philips Her2/neu IHC Digital Manual Read will now be marketed in the United States. More information, please visit www.philips.com/digitalpathology.
HercepTest™ is a semi-quantitative immunohistochemical assay for determination of HER2 protein. HercepTest™ are trademarks of Genentech, Inc. subject to licenses held by Dako Denmark A/S.
Source: Philips
































