What Is a Hot-Spot and Does It Matter?
Courtesy of Dr. Michael Grunkin, CEO, Visiopharm
What Is a Hot-Spot and Does It Matter?
 The importance of hot-spots
The importance of hot-spots
As discussed in the recent post on managing tumor heterogeneity, it is widely considered best practice to determine the Ki-67 proliferative index in a hot-spot of biomarker expression. A growing body of scientific evidence support that correct identification of hot-spots is essential to achieve sufficient interpretive accuracy for predictive and/or prognostic use [1,2]. Hot-spot scoring has recently been adopted in both Swedish and Danish clinical guidelines for breast cancer [3,4].
Heterogeneity is a general challenge
Heterogeneity with respect to expression levels is evident for many tissue biomarkers. Although it may be less obvious how to technically determine heterogeneity and hot-spots for membrane markers, or markers that express in several sub-cellular compartments, it is likely that the ability to visualize and quantify heterogeneity and identify hot-spots will be generally important to cancer research, drug development, biomarker validation, and in diagnostics. To further explore this, we developed a general research tool for quantification of tissue biomarkers, including generation of heatmaps of expression and identification of hot-spots..
The definition of a hot-spot
As an example, this general research tool was used to create an APP for visualizing heatmaps for Ki67 expression across entire tissue sections, and determine the location of hot-spots. Although this seems an intuitively appealing approach for managing heterogeneity, it generates new questions relating to the definition of a hot-spot. Clinical guidelines are usually defining a hot-spot as a square of fixed dimensions or recommend counting a certain number of cells around the hot-spot. For their presentation at the 29th European Congress of Pathology, Omanovic and Schönau were comparing five different approaches to defining a hot-spot as outlined in the figure below. Note how the hot spot location and area changes between methods, and that heatmap area and count can be defined along the iso-curve of the biomarker response.
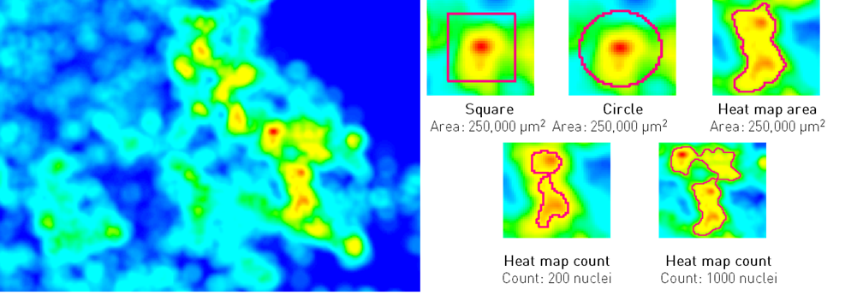
Need for a standardized approach
In the study above, they found that different definitions as well as area of hot-spots has a statistically significant impact on the proliferation index. Therefore, the fact that different guidelines and hot-spot definitions are adopted in clinical practice and for research purposes, also means that results cannot be compared. Thus, it will difficult to define generally applicable clinical standards (e.g. cut-offs) until there is a consensus on a unified / optimal definition of a hot-spot.
Clinical relevance
Apart from the need for standardization, there is also a need to understand to the impact of the observed differences on the predictive or prognostic power of tissue-based assays. At Karolinska University Hospital, Johan Hartman et. al. are currently exploring this in a study design with a longitudinal cohort, clinical outcomes and access to sequencing data. The outcome of this study will hopefully provide insights allowing for a recommendation of an optimal definition of hot-spots, for understanding the impact on diagnostic/prognostic accuracy, and to what extent a modern tissue-diagnostic approach provide statistically independent information compared to molecular methods.
References
- Gudlaugsson et. al.; Comparison of the effect of different techniques for measurement of Ki67 proliferation on reproducibility and prognosis prediction accuracy in breast cancer
- KVAST, Swedish breast cancer guidelines, 2018 (in Swedish)
- DBCG – Danish Guidelines, May 2017 (in Danish).
About the author
Michael Grunkin, PhD – Chief Executive Officer
Dr. Michael Grunkin is founder and managing director of Visiopharm. He received his PhD in image analysis from the Technical University of Denmark in 1993. His post-doctoral work was carried out on Massachusetts Institute of Technology and Harvard Medical School, where he combined a strong theoretical and practical background in image analysis with applications from the life-sciences. From 1996, he was the technical founder of two Danish companies developing advanced image analysis technology for diagnosing osteoporosis and various diseases manifesting themselves on the human retina. In 2001, he co-founded Visiopharm, which is focused on the development of general as well as application-specific image analysis solutions for the life-sciences.
































