A Multi-Institutional Study on Adoption of Automated Image Analysis for Programmed Death-Ligand 1 (PD-L1) expression by OptraSCAN Published in a Scientific Journal
OptraSCAN®, the leading end-to-end digital pathology solution provider announced that a research paper on ‘A Multi-Institutional Study to Evaluate Automated Whole Slide Scoring of Immunohistochemistry for Assessment of Programmed Death-Ligand 1 (PD-L1) Expression in Non–Small Cell Lung Cancer’ was published in ‘Applied Immunohistochemistry & Molecular Morphology’, the official publication of The International Society For Immunohistochemistry & Molecular Morphology.
Details about the study:
Assessment of programmed death-ligand 1 (PD-L1) expression is a critical part of patient management for immunotherapy. However, studies have shown that pathologist-based analysis lacks reproducibility, especially for immune cell expression. The purpose of this study was to validate reproducibility of the automated machine-based Optra image analysis for PD-L1 immunohistochemistry for both tumor cells (TCs) and immune cells.
“The inter-pathologist concordance seen in this study is similar to previously reported studies, where agreement is higher for evaluation of TCs than for immune cells. The present study shows that the Optra PD-L1 image analysis showed concordance with the pathologists’ immune cells,” said Abhi Gholap, Founder & CEO, OptraSCAN. He further added by saying, “This finding suggests promise for the use of automated WSI analysis assessment in non–small cell lung cancer for accurately generating scores for tumor and immune cell population.”
METHOD
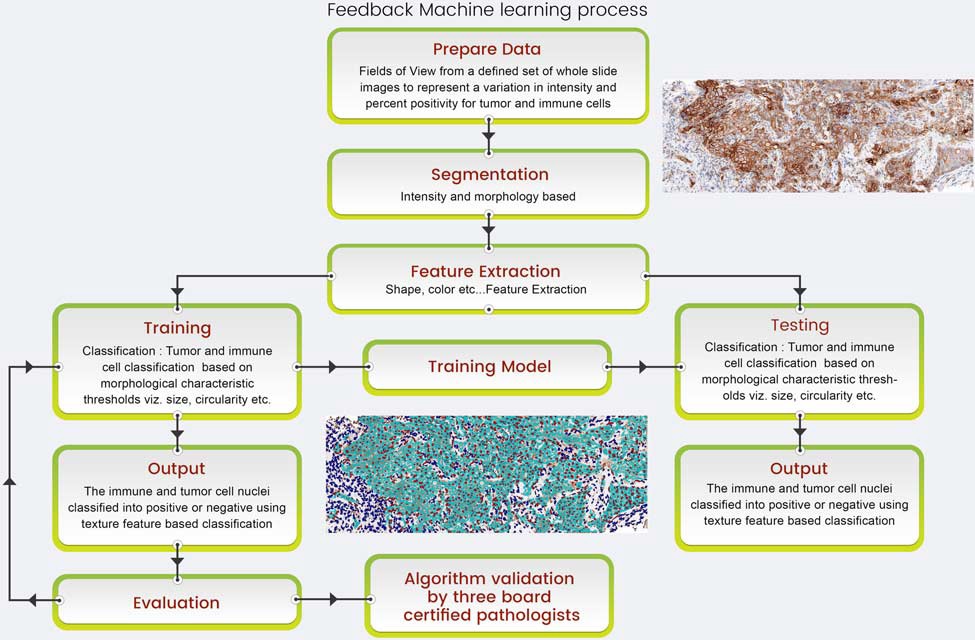
FIGURE 1. Optra PD-L1 algorithm flow chart showing the computing steps involved in the working of the algorithm.
RESULTS:
In efforts to evaluate the ability of the algorithm to correctly predict positive and negative membrane PD-L1 expression as an analog for sensitivity and specificity, respectively, on tumor and immune cells, the criterion standard was the pathologist’s digital manual reads. The accuracy of the algorithm to detect membrane positivity was 88% and 84% for tumor and immune cells, respectively. The algorithm performed well with; 0.8 (area under curve) for tumor and 0.7 (area under curve) for immune cells.
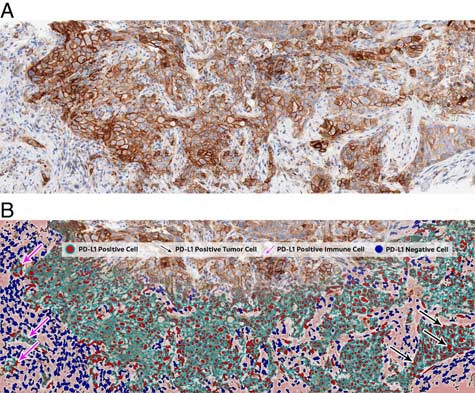
FIGURE 2: A, PD-L1 immunohistochemistry stained raw digital image as input data for image analysis. B, Markup image generated to illustrate image analysis results.
“A challenge for immunohistochemistry assays is that they are optimized to provide a binary outcome, but, in reality they detect biomarkers that are innately expressed as a continuous variable. This scenario poses a problem for pathologists, who are asked visually to assess target protein expression across a population of many thousands of cells in a tissue section and to arrive at a score for TCs, or any other admixed cell population (such as lymphocytes and/or macrophages), to give a result that can predict a response to the targeted therapy,” says Dr. Clive Taylor, Chief Medical Officer, OptraSCAN. “Rapid technological advances and the advent of computer-aided platforms have permitted development of machine-based scoring algorithms that have capabilities in overcoming these challenges, and I am happy that OptraSCAN is at the forefront of such technology development,” he added.
About OptraSCAN®:
An ISO 13485 certified company and CE marked whole slide scanners for IVD use, OptraSCAN® has developed world’s first ‘On-Demand Digital Pathology’ System; focused on delivering fully integrated, affordable systems & solutions. These serve as the perfect tool for transition from conventional microscopy to Digital Pathology for the effective acquisition of whole slide images, viewing, storing, real time sharing, and reporting via On-Demand or outright purchase model. Follow Us on LinkedIn and Twitter
Media Inquiries Contact:
Shruti Soman | Manager – Marketing Communications | 91.20.66540900 x 231 | s.soman@optraventures.com
Source: Optrascan
































