November 19, 2019
Tissue Classification To Direct Image Analysis of IHC Digitized Slides
 Marie-Louise Loupart, Digital Pathology Software Specialist
Marie-Louise Loupart, Digital Pathology Software Specialist
- Are you frustrated by all that laborious manual annotation to make sure only your ROIs are analyzed? Does it feel like your ‘automated’ image analysis isn’t quite so automated after all?
- Do you need to separate out tissue types to compare and contrast distinct cell types? Are all those immune cells you’ve identified by IHC actually within the tumor micro-environment?
- Can you see your ROI in the H&E but having trouble locating the corresponding area in the IHC slide? Or maybe you are like me (not a pathologist): confident with the IHC, but tend to puzzle over the H&E.
- For Today, Machine Learning is a good aid for oncology and many other histopathology applications. Lets consider some examples for ensuring the correct ROIs are analysed:
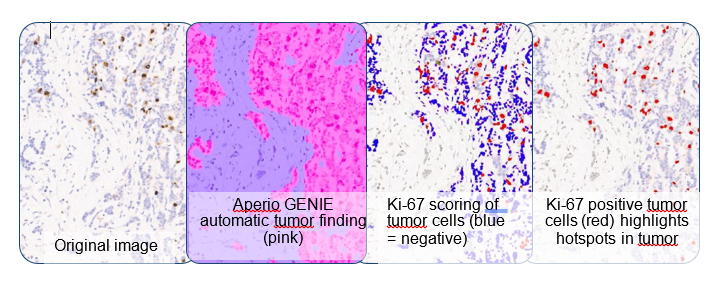
Example #1: Ki-67 hotspot tumor analysis is a 2-step process:
- Identify the tumor ROIs
- Score for Ki-67 in the tumor cells
- Sounds easy? Not with invasive tissue that by its nature will be interspersed with the normal tissues. Use a tumor-finding classifier. It is easier to score Ki-67 with a nuclear counting algorithm. Ki-67 tumor hotspots will stand out (below right).
- % Ki-67 positive = 11% tumor cells, but only = 4% all cells (tumor & normal).
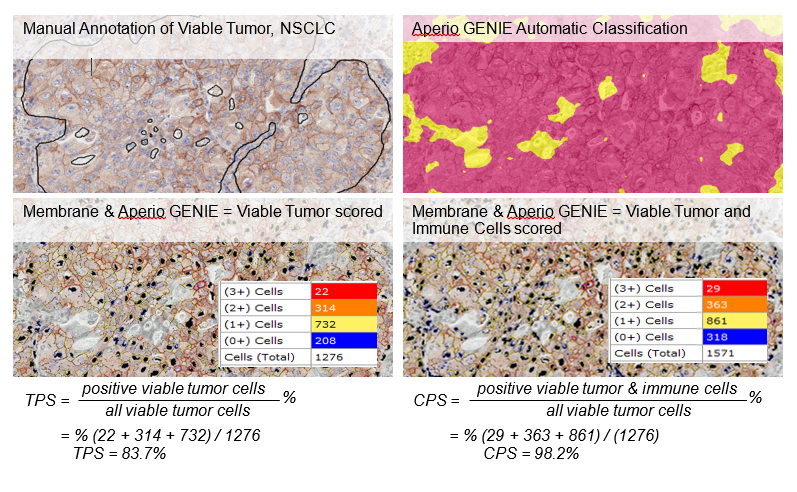
Example #2: PD-L1 interpretation is fraught with complexity:
- Guidelines: membrane not cytoplasmic staining, count ³ 100 viable tumor cells, separate immune cells, ignore necrotic cells, ignore variable staining of tumor cells
- Broadly, there are 2 scoring strategies:
- Tumor Proportion Score (TPS) – widely adopted with manual scoring or an algorithm, but it can be hard to exclude unwanted cells
- Combined Positive Score (CPS) – subject of intense scrutiny as a more reliable indication for NSCLC only
- Aperio GENIE can help by finding viable tumor ROIs. A good membrane algorithm can then filter out smaller immune cells to generate a TPS for lung, melanoma and other tumor sites. CPS for NSCLC takes a bit more work: the membrane algorithm is run twice: with and without scoring immune cells in the tumor ROI.
If that’s Today… what about Tomorrow? Smart AI classifiers are coming into play. The danger is the higher volume of examples they need. This could mean DL classifiers will be hard to produce for individual/specialist assays. I strongly suspect that ML will still be around tomorrow working alongside DL. You get to chose the tool to suit your needs: fast-build local classifiers for your pet project; slow-build global classifiers for diagnostic support and large-scale projects. I like choices…
Source: Leica Biosystems
© 2023 Tissuepathology.com. All rights reserved. Millennial Consulting. | Web Design by Zealth Digital Marketing
































