INDICA LABS’ HALO AP® PLATFORM ACHIEVES CE-IVD CERTIFICATION FOR USE IN PRIMARY DIAGNOSIS
 Albuquerque, NM, 10 January, 2022 – Indica Labs, a leading provider of computational pathology software and services, is proud to announce that following ISO13485 certification, HALO AP® has received a CE Mark for use in primary diagnosis within the European Economic Area, Switzerland, and the UK.
Albuquerque, NM, 10 January, 2022 – Indica Labs, a leading provider of computational pathology software and services, is proud to announce that following ISO13485 certification, HALO AP® has received a CE Mark for use in primary diagnosis within the European Economic Area, Switzerland, and the UK.
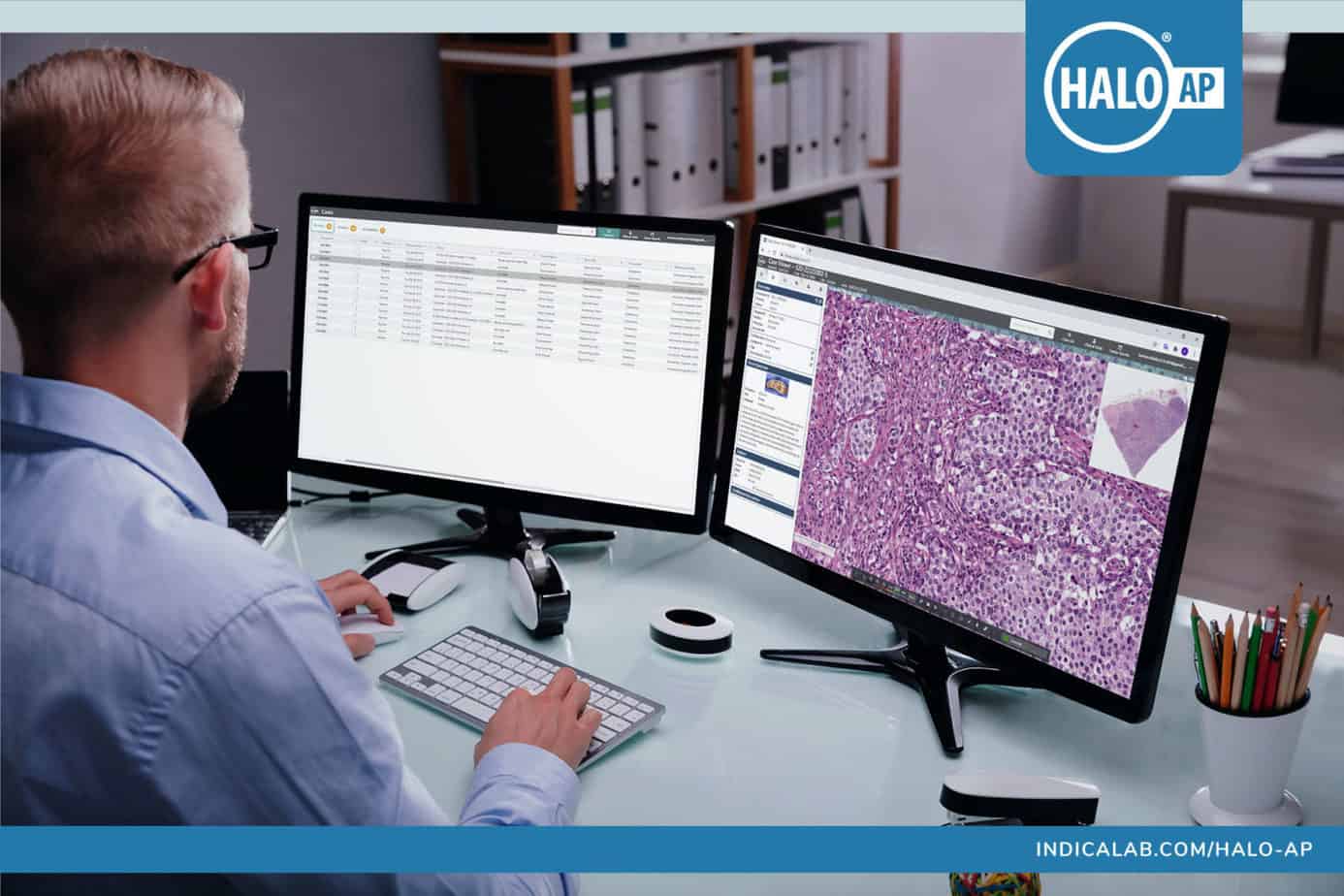
The HALO AP platform was developed by Indica Labs to address the specific needs of anatomic pathology labs by supporting a wide range of tissue-based workflows, including primary diagnosis, secondary consults, tumor boards, clinical trials, synoptic reporting, quantitative analytics, and AI.
Anatomic pathology is undergoing a major sea change as AI and computational tools become increasingly intertwined with routine digital workflows, and cloud-based technologies become the norm rather than the exception. Supporting image formats from all leading whole slide scanner manufacturers, and with flexible deployment and integration options, HALO AP was built from the ground up to enable these emerging technologies and future proof anatomic pathology labs moving forward.
HALO AP incorporates state-of-the-art workflow management tools allowing users to standardize reporting steps by defining tasks and dependances, including the ability to easily integrate quantitative analytics and AI tools built using HALO®, HALO AI™ and third-party platforms. Tasks can be fully automated or delivered in a stepwise, guided fashion simplifying training and deployment. Integrated workflows are locked, secured, and audited to prevent tampering or misuse.
With APIs to support connections to LIS/LIMS or other third-party systems, as well as flexible on-premise and cloud deployment options, HALO AP can integrate seamlessly within any organization’s existing IT infrastructure. For laboratories without an existing LIS/LIMS, HALO AP can act as a fully functional, standalone case and image management system.
“Indica Labs strongly believes in providing pathologists with easy-to-use, AI-enabled tools to meet their increasingly complex, high-volume workloads. Achieving CE-IVD certification for primary diagnosis is an important first step for HALO AP to help unlock the potential of digital pathology in health systems across Europe and the UK,” said Eric Runde, Chief Operating Officer at Indica Labs.
The CE-IVD HALO AP 1.3 is already in use at Nottingham University Hospitals NHS Trust (NUH), one of the largest NHS trusts in England processing approximately 340,000 slides per year. HALO AP is integrated with existing hospital systems and used alongside Hamamatsu scanners to provide management and viewing of scanned slides for primary diagnosis, secondary consultations, tumor boards, remote reporting and multi-disciplinary team meetings.
To learn more about HALO AP, contact us at info@indicalab.com for a full demonstration.
































