March 10, 2021
Huron Receives Two Regulatory Approvals for TissueScope iQ
 TissueScope iQ Receives Health Canada and CE-IVD
TissueScope iQ Receives Health Canada and CE-IVD
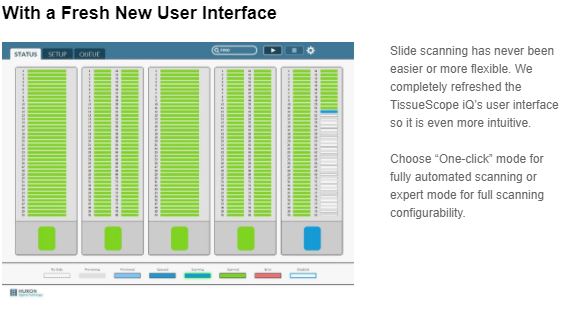
Over the past month, Huron’s 400 slide TissueScope iQ achieved two important regulatory approvals, Health Canada and CE-IVD for Europe. With these certifications, Huron is accelerating its entry into the diagnostic pathology market in key geographies globally to help laboratories keep pace with the rising cancer burden. TissueScope iQ joins TissueScope LE and TissueScope LE120 with these regulatory approvals.

Source: Huron Digital Pathology
© 2023 Tissuepathology.com. All rights reserved. Millennial Consulting. | Web Design by Zealth Digital Marketing