Indica Labs: Low and Ultralow HER2 Expression in Breast Cancer is Now Detectable by Indica Labs’ Breast IHC AI
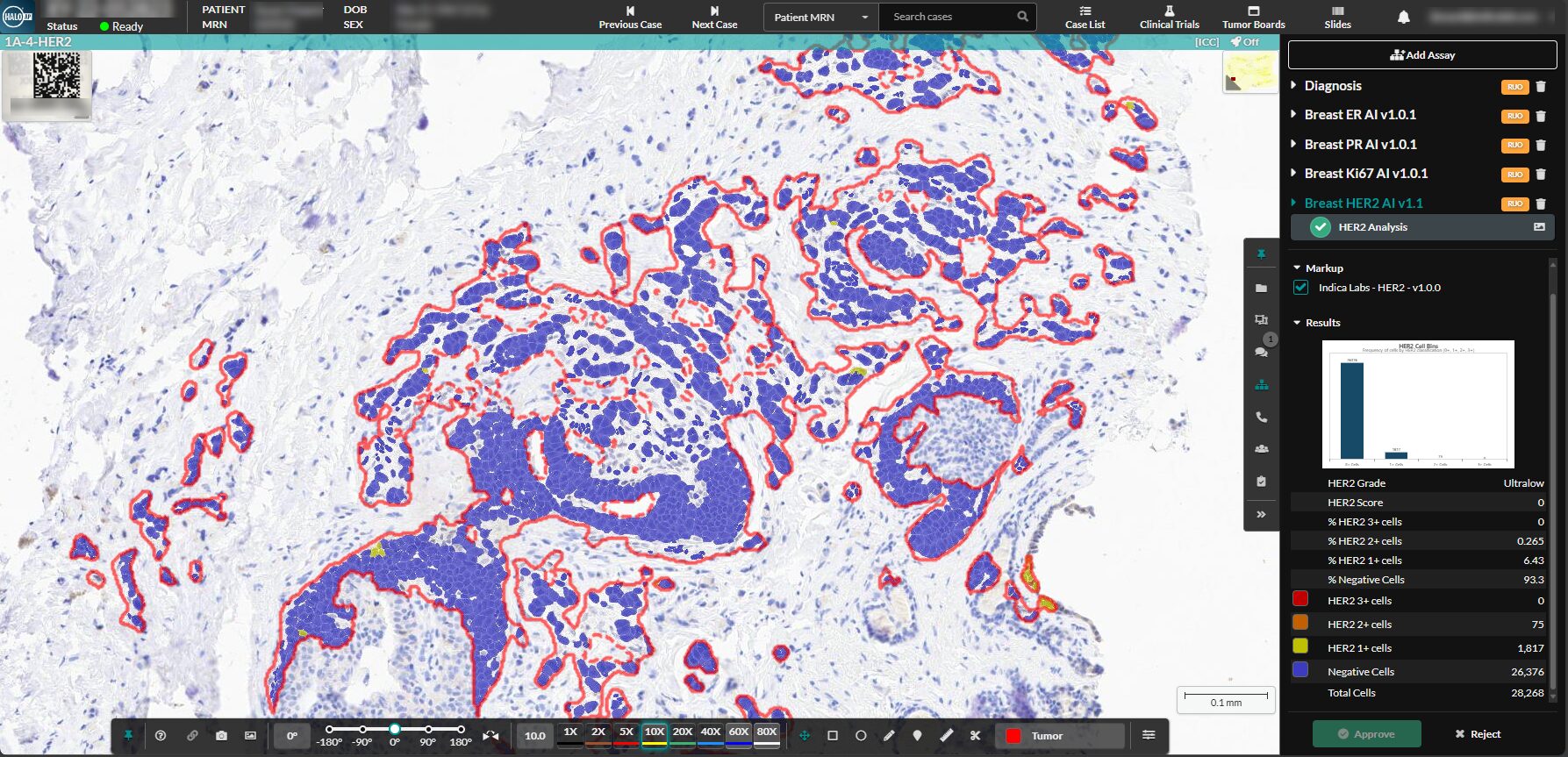 Albuquerque, NM, April 30, 2025 – Indica Labs, the leading provider of AI-powered digital pathology solutions, announces today an update to their Breast IHC AI suite, an image analysis tool used for the quantification of HER2, ER, PR, and Ki67 within AI-identified, tumor regions, of breast cancer tissue specimens. The HER2 algorithm will now include functionality for the detection of low and ultralow HER2 expression in breast cancer.
Albuquerque, NM, April 30, 2025 – Indica Labs, the leading provider of AI-powered digital pathology solutions, announces today an update to their Breast IHC AI suite, an image analysis tool used for the quantification of HER2, ER, PR, and Ki67 within AI-identified, tumor regions, of breast cancer tissue specimens. The HER2 algorithm will now include functionality for the detection of low and ultralow HER2 expression in breast cancer.
The HER2 algorithm categorizes HER2 expression as 0,1+, 2+, 3+ which has been historically interpreted as negative or positive based on current guidance from ASCO/CAP guidelines; however, due to recent data, the interpretation of HER2 expression in breast cancer is now understood as a spectrum of immunohistochemistry (IHC) membrane staining intensity, where even the most discrete levels of staining are considered medically significant. The clinical importance of HER2-low is in response to emerging clinical trial data in which HER2 directed antibody drug conjugates (ADCs) were effective at targeting tumor cells with low levels of expression. This recent change in the clinical understanding of HER2 expression has prompted the update to Indica Labs’ HER2 algorithm which now has the functionality to recognize and automatically quantify HER2 low and ultralow membrane staining intensity. The platform displays a HER2 IHC Score of 0,1+, 2+, or 3+ with a corresponding Grade of Negative, Ultralow, Low, 2 or 3. This AI-powered tool will help pathologists accurately and efficiently interpret biomarker expression at even the lowest levels of detection.
The software automatically reports the percentage of positive cells and biomarker scores at the slide level, along with image analysis masks which can be viewed in the HALO AP® platform. HALO AP® is trusted for its robust suite of tools for annotation, collaboration, and image analysis. The platform offers interoperability and compatibility with a wide range of scanners, file types, and clinical data systems, ensuring flexibility and ease of use in diverse laboratory environments.
Each breast biomarker assay has built-in artifact and benign epithelial exclusion as well as tumor detection. These pre-processing steps ensure biomarker analysis is performed accurately and consistently each and every time. Tumor cells are analyzed for expression of HER2, ER, PR, and Ki67 and a comprehensive set of results and markups are generated for each image, including clinical score and percent positivity. Additionally, the end user can approve an assay, lock it, and sign it out to prevent unintended changes. This automated workflow decreases subjectivity, standardizes biomarker scoring, and safeguards the pathologist’s work.
“Indica Labs is dedicated to advancing medical research and improving clinical workflows,” said Eric Runde, Chief Operating Officer at Indica Labs. “Breast IHC AI uses the power of AI to automate biomarker expression analysis, facilitating rapid interpretation of results while allowing pathologists to maintain full control of the final scoring and reporting process. Our AI-powered tools streamline the pathologist’s workflow so more time can be spent on the most challenging cases.”
External validation for breast cancer biomarkers HER2, ER, PR, and Ki67 were assessed for clinical performance and generalizability using the percentage of agreement between the Brest IHC AI algorithm score and clinical data, resulting in an agreement as high as 96% for some biomarkers. The validation data supports the algorithms’ ability to provide consistent, standardized measurements for the accurate analysis of breast cancer biomarkers.
For more information about Breast IHC AI, HALO Clinical AI Solutions, and how these algorithms can enhance efficiency and accuracy in your laboratory, contact info@indicalab.com.
Breast IHC AI is For Research Use Only and not intended for clinical diagnostic use. Breast IHC AI is accessed via the HALO AP® enterprise digital pathology platform.
HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP® is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP® provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.
About Indica Labs:
Indica Labs is the global leader in AI-powered digital pathology software and services. Our flagship HALO® and HALO AI platform revolutionizes quantitative evaluation of whole slide images. HALO Link provides collaborative image management while HALO AP® and HALO AP Dx deliver enterprise digital pathology for primary diagnosis with regulatory clearances in multiple markets. Through a commitment to open pathology, performance, scalability, and ease-of-use, we help pharma companies, diagnostic labs, hospitals, research organizations, and Indica’s own Cloud and Pharma Services make discoveries and diagnoses that transform patient care and scientific discovery.
Media Contact:
Eric Runde
erunde@indicalab.com
SOURCE: Indica Labs
































