Indica Labs Announces First Webinar of 2026
 Indica Labs: Learn how HALO and HALO AI are helping expand our understanding of immune regulation by different subtypes of cancer-associated fibroblasts!
Indica Labs: Learn how HALO and HALO AI are helping expand our understanding of immune regulation by different subtypes of cancer-associated fibroblasts!
Highly multiplexed imaging reveals CAF-immune cell niches in cutaneous carcinomas
Date: 22 January 2026
Time: 8:00 – 9:00 PST | 11:00 – 12:00 EST | 16:00 – 17:00 GMT
Location: Webinar
Summary
Cancer-associated fibroblasts (CAFs) shape the tumor microenvironment (TME), influencing immune cell (IC) infiltration and anti-tumor activity. Three major CAF subtypes are identified in cutaneous carcinomas. Immunomodulatory CAFs (iCAFs), enriched in late-stage tumors, express cytokines and chemokines that promote IC recruitment and activation. Matrix CAFs (mCAFs) produce extracellular matrix and ensheath tumor nests, restricting IC entry. Myofibroblast-like CAFs (myoCAFs) resemble pericytes and contractile fibroblasts and likely support tissue remodeling and tumor vasculature.
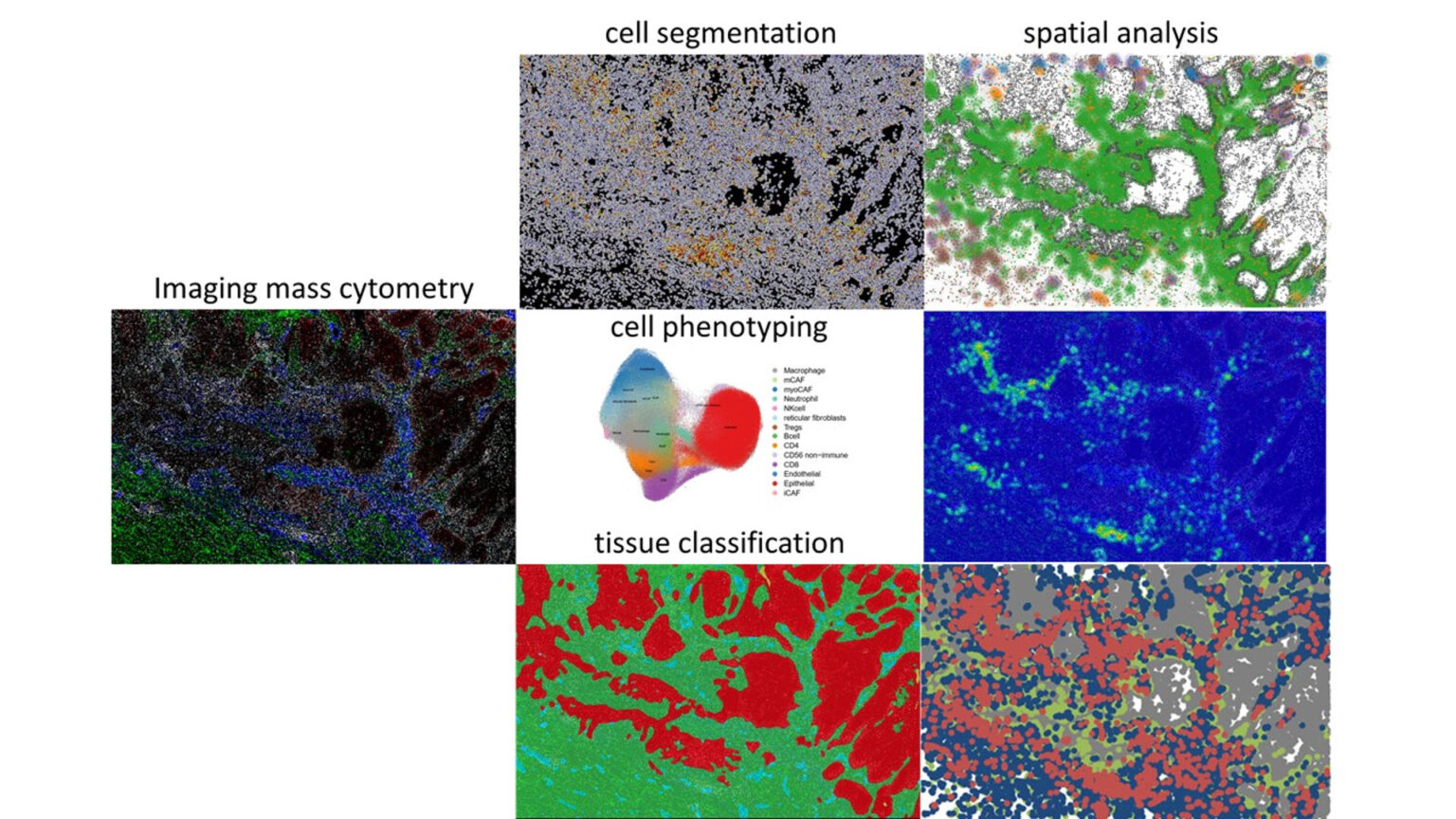
Keratinocyte cancers, including basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs), were profiled by Imaging Mass Cytometry using a panel targeting major myeloid, lymphoid and CAF subsets, including activation and exhaustion markers. Image analysis, clustering, and spatial mapping were performed with HALO, Seurat, and Squidpy, enabling high-resolution definition of CAF niches.
SCCs harbored more immune cells than BCCs, particularly lymphocytes. Tumor immune phenotypes—immune-desert, -excluded, and -inflamed—correlated with CAF composition: inflamed SCCs were enriched in iCAFs, whereas immune-excluded BCCs and SCCs contained more mCAFs and myoCAFs; infiltrative BCCs were particularly enriched in myoCAFs. Spatial analysis mapped iCAFs adjacent to lymphocytes, while myoCAFs formed fibroblast-rich stromal regions largely devoid of immune cells. These findings indicate that specific CAF subtypes differentially regulate immune infiltration and activation, suggesting that targeting specific CAF subsets could enhance immunotherapy efficacy in cutaneous keratinocyte carcinomas and could be possibly translated to other keratinocyte cancers.
Learning Objectives
- Learn how to analyze highly multiplexed stainings in HALO
- Learn about the workflow applied in the study: Cell segmentation, tissue classification, cell phenotyping in R, import of cell phenotypes back into HALO to conduct quality control and spatial analysis.
- Learn how cancer-associated fibroblast niches influence immune cell activation in skin cancer
Presenter
Bertram Aschenbrenner, PhD
Post-Doctoral Fellow
Lichtenberger Laboratory, Medical University of Vienna
Dr. Bertram Aschenbrenner studied molecular biology at the University of Vienna, Austria and performed his master studies in the laboratory of Manuela Baccarini analyzing CRAF – Rok-α interactions. During his PhD studies in the research group of Ira-Ida Skvortsova at the Medical University of Innsbruck, he dissected radioresistance mechanisms of mesenchymal breast carcinoma cells.
In his current role as a post-doctoral fellow in the lab of Beate Lichtenberger at the Medical University of Vienna, Bertram uses imaging mass cytometry, 2D and 3D cell culture systems, and skin cancer organoids harboring native tumor stroma to investigate different immunomodulatory functions of cancer-associated fibroblasts.
SOURCE: Indica Labs
next article
Deer Park, IL, February 11, 2026 – Leica Biosystems, a Danaher company and a global leader in anatomic pathology solutions, is redefining histology workflows with the global launch of the Leica CM1950 Cryostat with DualEcoTec Cooling...
































