Visiopharm Fully Automated APP for Ki67 Assessment in Breast Cancer CE Marked for In-Vitro Diagnostic Use in Europe
Exciting news from Visiopharm – their Ki67 diagnostic App has received the CE mark for in-vitro diagnostic use in Europe.
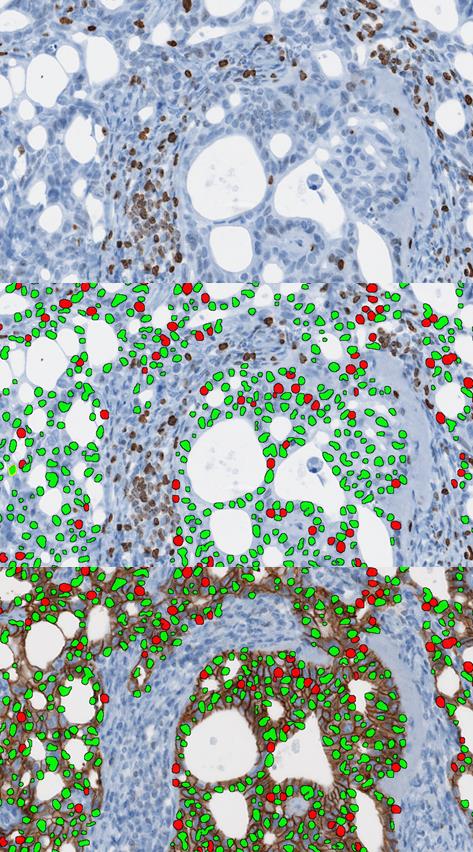 The Virtual Double Staining (VDS) Ki67 module for breast is a novel, patented and now CE-marked diagnostic APP intended for automated tumor/stroma separation and computation of the Ki67 labelling index for the identified tumor cells.
The Virtual Double Staining (VDS) Ki67 module for breast is a novel, patented and now CE-marked diagnostic APP intended for automated tumor/stroma separation and computation of the Ki67 labelling index for the identified tumor cells.
The Visiopharm VDS-Ki67 APP was developed in collaboration with the independent scientific organization Nordic Immunohistochemical Quality Control (NordiQC). The APP has been formally validated across several Danish and international pathology labs. The approach implemented in this IVD APP accomplishes two things: It significantly improves reproducibility as well as productivity for Ki67 assessment in breast cancer.
“What makes a big difference to us is that the underlying technical principles allow us to achieve a high level of automation in the quantification process, including tumor/stroma separation. Achieving this level of automation for Ki67, and also for other biomarkers, will allow us to improve productivity in the pathology lab at a level that justifies the investment in Digital Pathology for routine diagnostics,” stated Prof. Torben Steiniche at Aarhus University Hospital.
“In a recent study we compared the Virtual Double Staining method for Ki67 in breast cancer to manual scoring data from 126 pathology labs. We found considerable inter-lab variability in the manual scoring. Our data suggests that automated removal of stromal cells, followed by automated quantification, significantly reduces reader variability. Standard image analysis, requiring manual removal of stromal components, will likely not lead to the same improvements in reproducibility. In addition, it would have a negative impact on productivity. To the best of my knowledge, Virtual Double Staining is the only principle that allows us to achieve tumor/stroma segmentation in an automated and immediately verifiable way, which can readily be implemented in a typical pathology lab,” says Prof. Mogens Vyberg, Director of NordiQC.
“We are validating all CE-IVD APPs, including VDS-Ki67 for breast, across the major stainer and scanner platforms. The performance data we have achieved is very encouraging. We have seen very high correlations between manual and automated counting, with an R-square varying between 0.97 and 0.98 across manual scores from different pathologists at two major pathology labs. Looking at the validation of the APP and when dichotomizing patients for treatment decisions we saw agreements with manual reading across three other pathology labs varying between 83% and 91%,” stated Johan Doré, CTO at Visiopharm. The package insert for the VDS-Ki67 APP is available upon request.
“We are looking at a series of additional CE-IVD releases later this year. Supported by the Market Development Fund, we are now in the process of implementing the full breast panel across almost 20 European hospitals in fully LIS driven workflows. The two most important goals are improvement of data quality and productivity. Both aspects are core to demonstrating a solid business case for adoption of digital pathology in routine diagnostics. This will obviously require integration of additional markers and tumor panels, that are currently undergoing development and validation,” stated Michael Grunkin, CEO at Visiopharm.
About Visiopharm
Over the past 13 years, Visiopharm image analysis and stereology software has become the preferred Quantitative Digital Pathology solution for healthcare, life science, and biopharmaceutical organizations all over the world. Our software is featured in over 600 scientific publications, and is compatible with leading slide scanner manufacturers, data management software, and a wide variety of microscopes and cameras. Visiopharm has grown into an international business with over 400 customers in more than 30 countries. Our growing network of authorized distributors and integration partners support the growth of Visiopharm solutions on several continents including North America, Europe and Asia. Visiopharm is headquartered in the Medicon Valley of Denmark and has a North America office in Broomfield, Colorado.

































