Join Visiopharm at USCAP 2017 and learn about how we are transforming pathology! Come see demonstrations of
- Analysis of PD-L1 and CD Markers within the Tumor Micro-Environment
- Visualize and quantify biomarker expression with heatmaps and automated hot-spot detection
- The new, ultra-fast viewer which can be integrated into your digital pathology workflow
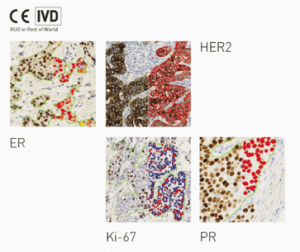 Visit our booth to compete head-to-head against Visiopharm’s quantitative HER2* and Ki-67 APP algorithms. Simply tell us how you like to assess Ki-67, global and/or hotspots, and how you currently process inconclusive HER2 cases. Then test yourself with a real-world case and see how the diagnosis may change.
Visit our booth to compete head-to-head against Visiopharm’s quantitative HER2* and Ki-67 APP algorithms. Simply tell us how you like to assess Ki-67, global and/or hotspots, and how you currently process inconclusive HER2 cases. Then test yourself with a real-world case and see how the diagnosis may change.
- Identify the hotspot and tell us the Ki-67 index
- Divide inconclusive HER2 cases in 1+ and 3+ categories
The Visiopharm APPs for Breast Cancer Ki-67, HER2, ER and PR are validated for in vitro diagnostic use (CE-IVD) in Europe and are currently for Research Use Only outside Europe.
Visiopharm software is designed to give pathologists an efficient, ergonomic workplace; supported by the Visiopharm Viewer. Our viewer allows windows to be distributed across multiple screens, works with multiple input devices, and eliminates the stress factor of slow and tiled screen updates; which is often common with other whole slide image viewers. Read more about our quantiative digital pathology solutions here. Visit us at booth #606 at USCAP to learn more and see it in action!
*HER2 slides are concordent with FISH
Come visit Visiopharm at USCAP 2017! Challenge the Computer and see the largest, fastest growing APP Center in the industry at booth #606!

































