OptraSCAN® Expands Global Footprint Through New Distributor Partnerships
~ Creating mutually beneficial partnership with distributors
to strengthen sales channel in US, Europe, Africa and Asia ~
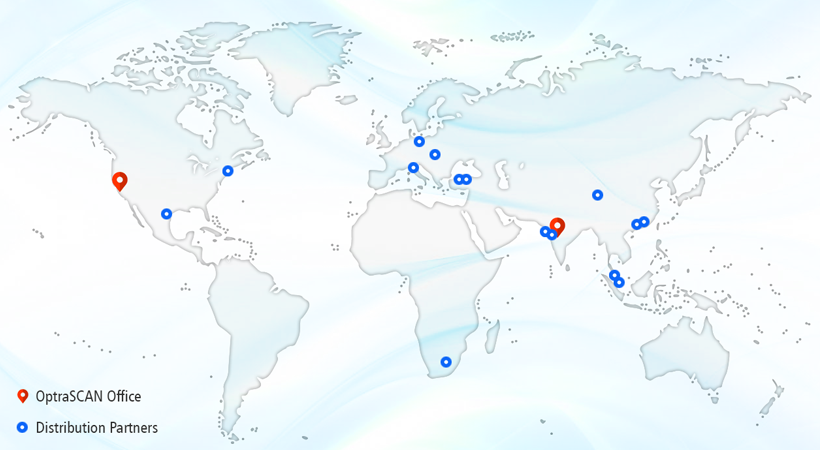
 San Jose, CA, November 13th, 2019: OptraSCAN®, the leading on-demand digital pathology solution provider today announced their plans for global expansion through distributor partnerships across the globe. Till date, OptraSCAN has signed strategic distribution partnership agreement with 12 channel partners to broaden the reach of their digital pathology systems & solution to US, Europe, Africa and Asian countries.
San Jose, CA, November 13th, 2019: OptraSCAN®, the leading on-demand digital pathology solution provider today announced their plans for global expansion through distributor partnerships across the globe. Till date, OptraSCAN has signed strategic distribution partnership agreement with 12 channel partners to broaden the reach of their digital pathology systems & solution to US, Europe, Africa and Asian countries.
OptraSCAN select their partners based upon their region-based sales and support, their commercial and technical capabilities to adopt OptraSCAN’s products and solutions and other measures. Basis this criterion, here’s the list of authorized distribution partners:
- USA: Nikon Instruments, Inc. and Meyer Instruments, Inc.
- Europe: Optiprime Dx SRL in Italy, HPST Ltd. in Czech Republic, Perlan Technologies in Poland
- South Africa: Healthcare IT
- Asia: Lawrence & Mayo and Labindia Instruments in India, PathVision Sdn Bhd in Singapore & Malaysia, SEM group, Doku Diagnostics in Turkey and APG Bio Ltd. in China
OptraSCAN is a known global provider of affordable digital pathology systems and solutions and as part of the partnership agreement, the distributors will have access to sell OptraSCAN’s many variants of small footprint, low and high throughput scanners which are convenient for brightfield, fluorescence, and multi-modality whole slide imaging. These scanners are accompanied with OptraSCAN’s proprietary software such as IMAGEPath®, image management system and TELEPath™, comprehensive telepathology solution. Along with this, OptraSCAN has also developed various AI & ML based image analysis solutions for nuclear & membrane marker analysis, immuno-oncology, prostate cancer analysis and cytology.
“The term ‘digital pathology’ in many developing as well as few developed countries is still novel, and we wish to tap these regions with our affordability quotient. Considering this, we have widened our distributor network around the globe to offer our solutions to the end-users who want an easy-to-use, compact scanning systems and cutting-edge solutions that will allow them to quickly adopt digital pathology; without burning a hole in their pocket,” said Abhi Gholap, Founder & CEO, OptraSCAN®.
To know more about our distribution network or partnership opportunities, write to us at info@optrascan.com
About OptraSCAN®:
An ISO 13485 certified company and CE marked whole slide scanners for IVD use, OptraSCAN® has developed world’s first ‘On-Demand Digital Pathology’ System; focused on delivering fully integrated, affordable systems & solutions. These serve as the perfect tool for transition from conventional microscopy to Digital Pathology for the effective acquisition of whole slide images, viewing, storing, real time sharing, and reporting via On-Demand or outright purchase model. Follow Us on LinkedIn and Twitter
Media Inquiries Contact:
Shruti Soman | Manager – Marketing Communications | 91.20.66540900 x 231 | s.soman@optraventures.com
Source: Optrascan.com

































