OptraSCAN to Expand into Digital Cytology Solution
- OS-SiA, an AI enabled scanner to revolutionize cervical cancer screening
- Complete Solution to Improve Medical Research and Digitize Clinical Workflow
 San Jose, California, January 31st, 2020 – OptraSCAN®, the leading On-Demand Digital Pathology solution provider, introduces its Digital Cytology Imaging solution that will aid pathologists to scan, index, and analyze the cytology slides through their next-gen smart scanner- OS-SiA. Healthcare providers deploying this technology stand to benefit rapid on-site evaluation for the adequacy of cytology samples.
San Jose, California, January 31st, 2020 – OptraSCAN®, the leading On-Demand Digital Pathology solution provider, introduces its Digital Cytology Imaging solution that will aid pathologists to scan, index, and analyze the cytology slides through their next-gen smart scanner- OS-SiA. Healthcare providers deploying this technology stand to benefit rapid on-site evaluation for the adequacy of cytology samples.
As workload increases in both complexity and volume, owing to a mounting number of cancer cases; pathologists are under pressure to deliver efficient services. OptraSCAN’s Digital Cytology Solution with its algorithmic methods driven by AI and ML technology strives to reduce the time needed to examine and assess the colossal number of samples individually.
“A major concern in the field of cytology is the volume of slides. Furthermore, medical practitioners do not have the luxury of time to evaluate each slide individually. To overcome this challenge, we are empowering pathologists with cost-effective Digital Cytology Imaging solution while increasing their productivity and better throughput. This highly-secure web-based solution also provides remote sharing of analyzed image, enabling new ways to collaborate with experts worldwide” said Abhi Gholap, Founder & CEO, OptraSCAN.
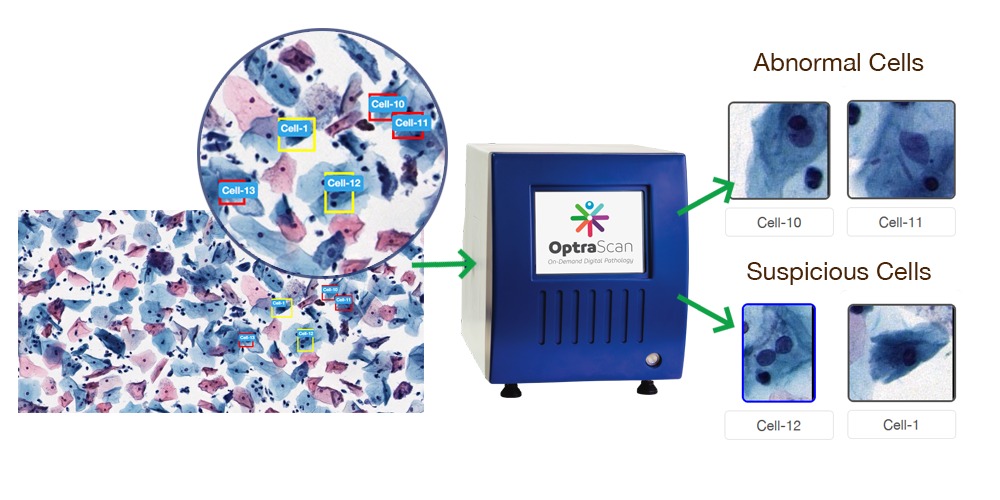
OptraSCAN’s advanced cytology solutions aims to reduce the workload of pathologists, increase and improve their efficiency. This is achieved by classifying the cytology slides into normal and abnormal during acquisition process of the digital image. The AI/ML based solution computes for the sample adequacy of the slide. The solution identifies and classifies the cells into normal and abnormal based on morphological, spatial and other features. As soon as the slide is digitized, instantaneously the analysis is available for the user.
OptraSCAN’s cytology solution incorporates differentiating technology-composite imaging (extended depth of field) which finds all pixels in focus from various Z plane images and stiches back to create a single layer composite image. This composite imaging technology is considerably an efficient viewing tool, which allows for swift and systematic screening of an entire image; and is set to disrupt the digital pathology market in Z stack imaging.
Advantages:
Automated classification of slides into normal and abnormal, by identifying abnormal cells.
Comparatively smaller file size than other multiplane images.
Quicker to acquire.
Effective and easy navigation for accurate interpretation, sharing and archiving.
Features:
- Classification of slides is done at the time of acquisition
- Fully automated multilayer scanning at different focal depths.
- Automated and User-configurable focal intervals for Z stacking.
- Ideal for rapid on-site evaluation for the adequacy of cytology samples (Rapid on-site evaluation (ROSE).
“The time associated with finding a few abnormal cells immersed in a sea of normal cells on a pap smear slide is accounting to mounting workload. With our unique expertise in digital pathology services, we are bridging this gap by scanning the tissue samples for adequacy and classifying them into abnormal and normal cells. Hence the pathologists only need to focus on slides that include any suspicious samples” says Dr. Clive Taylor, Chief Medical Officer, OptraSCAN.
With completing mass validation in early 2018 and receiving a positive response from leading cancer research centers, recognized hospitals, diagnostics laboratories, academic and research institutes operating in developing world, OptraSCAN is relentlessly working on reducing the disparities in the field of pathology by offering affordable digital pathology solutions.
About OptraSCAN, Inc.
OptraSCAN® are pioneers in the On-Demand Digital Pathology® System, focused on delivering fully integrated, affordable solutions that will maximize your return on investment and improve the performance of your pathology services. An ISO 13485 certified company and CE marked whole slide scanners for IVD use, OptraSCAN is working to eliminate the barriers to “Go Digital” no matter the size of the pathology lab, the lab’s throughput or global location.
OptraSCAN’s end-to-end digital pathology solution provides effective acquisition of whole slide images, viewing, storing, real time sharing, reporting and AI & ML based Image analysis solutions via On-Demand or outright purchase model. Follow Us on LinkedIn and Twitter.
Media Inquiries Contact:
Anjana Athanikar | Manager – Marketing Communications | 91.20.66540900 x 231 | a.athanikar@optraventures.com


































