 Akoya Collaborating with Agilent to Catalyze Precision Medicine
Akoya Collaborating with Agilent to Catalyze Precision Medicine
We recently announced a partnership with Agilent to develop multiplex diagnostic solutions for tissue analysis and to commercialize workflow solutions for these assays in the clinical research market. I’m thrilled that our organizations are collaborating to develop chromogenic and immunofluorescent multiplex assays that include spatial analysis for biopharma companies advancing precision cancer therapeutics. These assay solutions are designed to rapidly translate biomarker discoveries into clinical testing and in doing so, enable development of companion diagnostics based on “spatial signatures” that more accurately predict patient response and enable a more personalized approach to therapy selection.
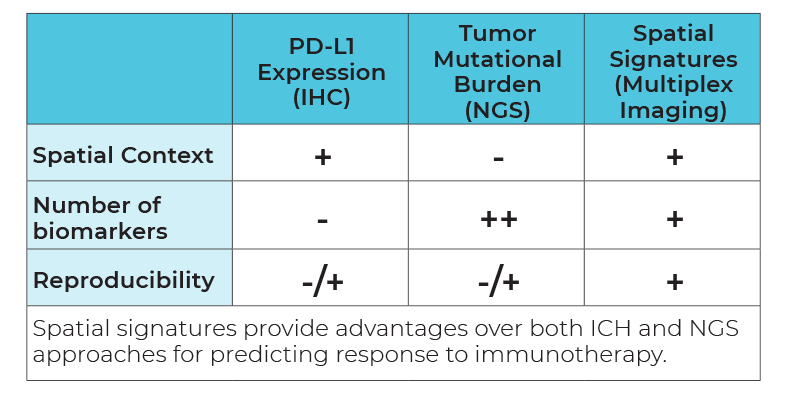
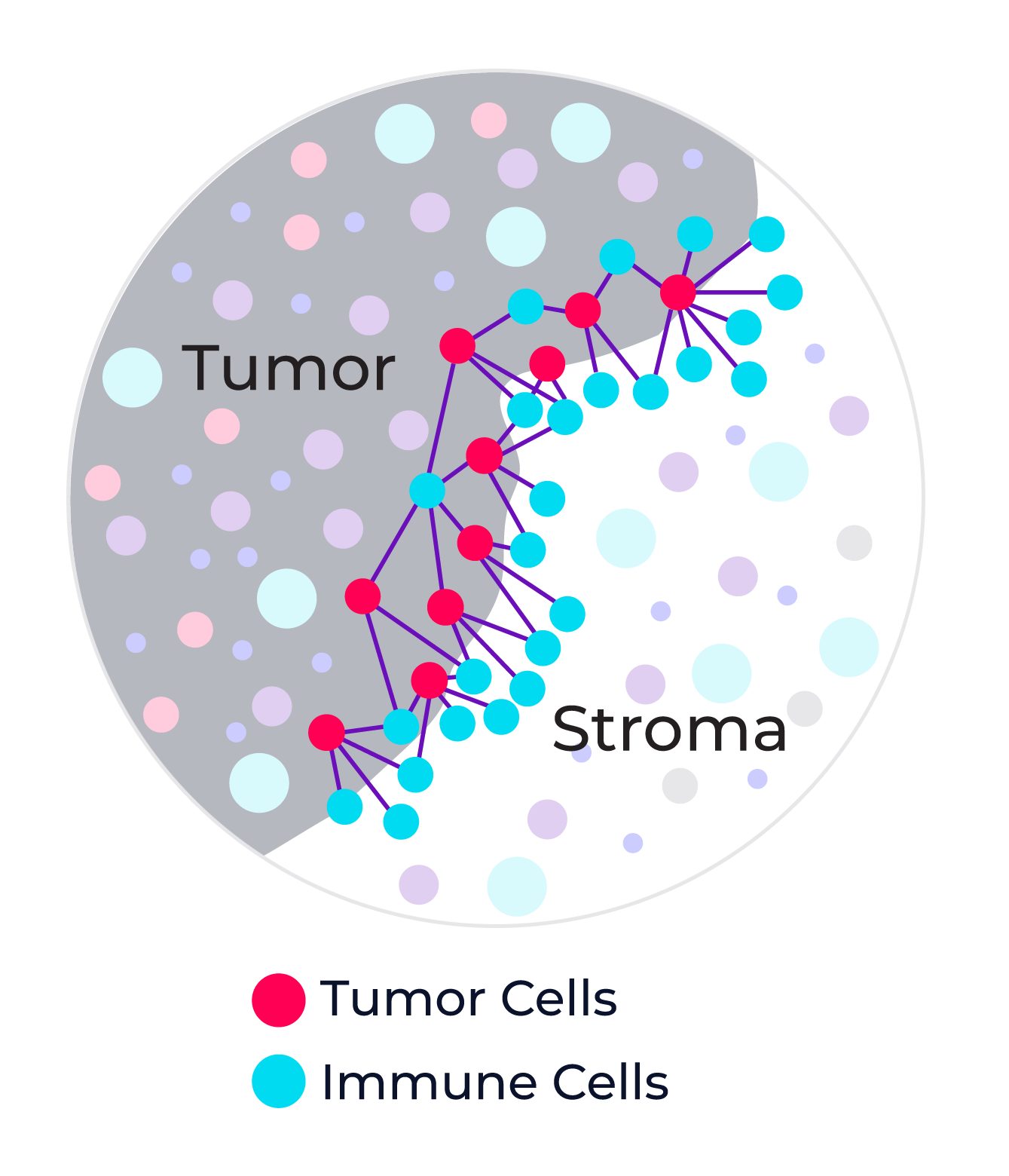
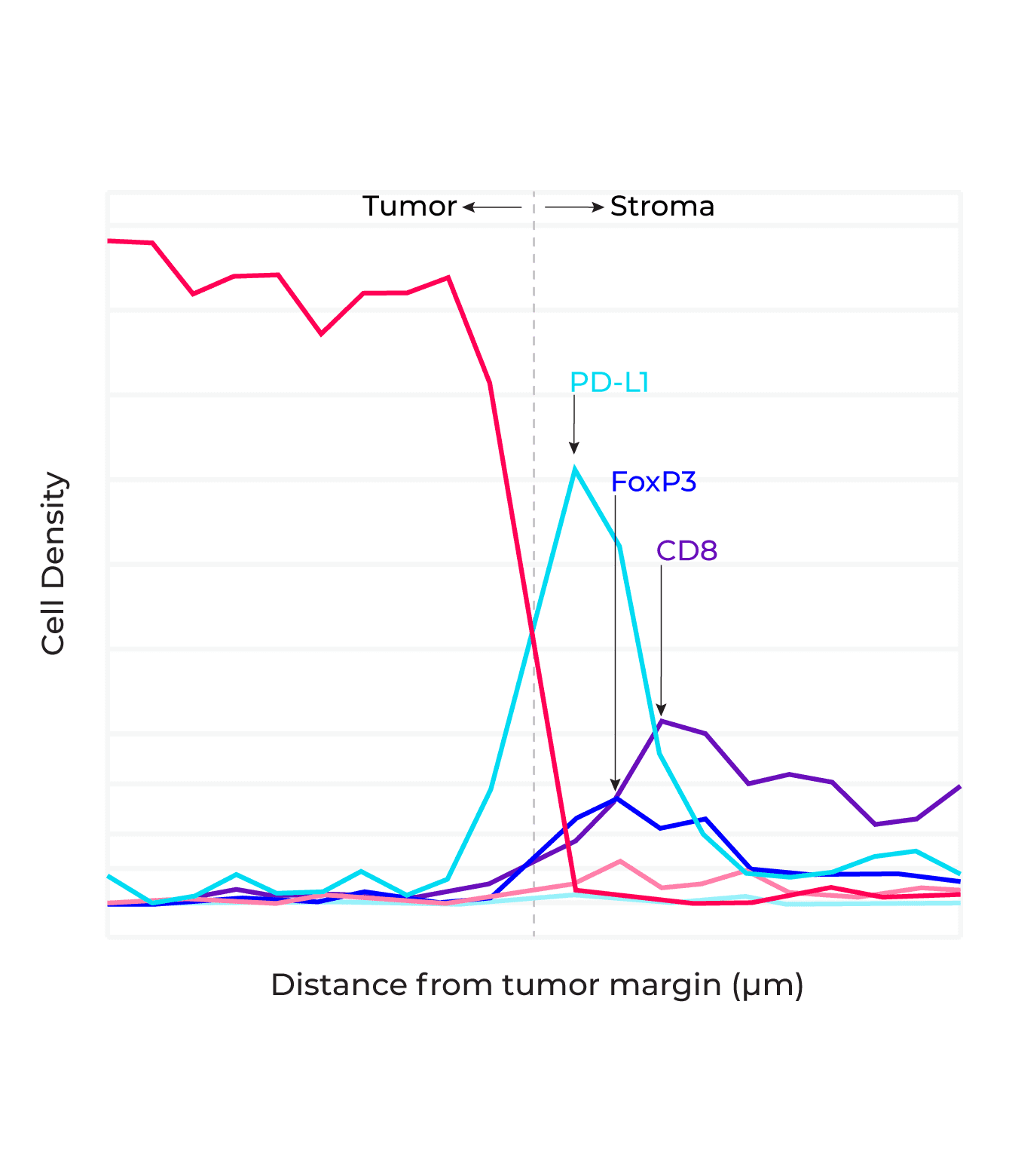
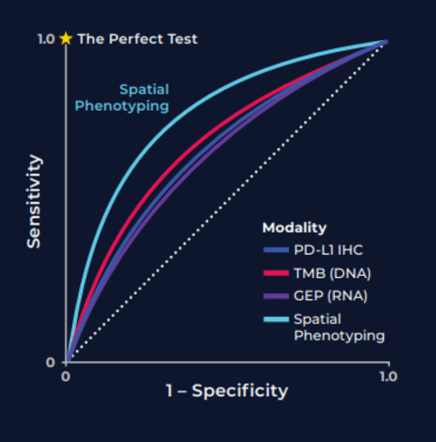
So, what are spatial signatures and how can they change the practice of cancer medicine? A signature in general represents a biological state unique to a specific tumor phenotype that takes the form of a pattern among multiple molecular markers, for example gene or protein expression, or genetic alterations such as mutations or fusions. A cancer spatial signature by extension is simply a signature where the spatial context of the cells are preserved and spatial information is incorporated into the signature definition.
Now we have a working definition of a spatial signature. But to truly understand why spatial signatures are important and why they represent a paradigm shift in immunotherapy predicitive biomarkers, its first necessary to review the current landscape for cancer biomarkers in general.